In an era where scientific research constantly evolves, we, at NeoTrident, are committed to empowering laboratories with innovative solutions that enhance productivity and streamline processes. The iLabPower Electronic Laboratory Notebook (ELN) app is designed to address the challenges researchers face in data management and documentation. With a focus on convenience and functionality, this application provides a suite of features tailored to meet the demands of modern research environments. Below, we delve into key aspects of the iLabPower ELN app that can significantly improve our research efficiency.
Voice Input Support for Enhanced Efficiency
One of the standout features of the iLabPower ELN app is its voice input support, which allows us to save valuable time when recording experimental notes. Instead of manually typing lengthy observations and data entries, we can simply speak our notes into the app. This feature not only streamlines the documentation process but also enables us to capture ideas and insights as they occur during experiments. By reducing the time spent on data entry, we can allocate more focus to the core tasks that drive our research forward.
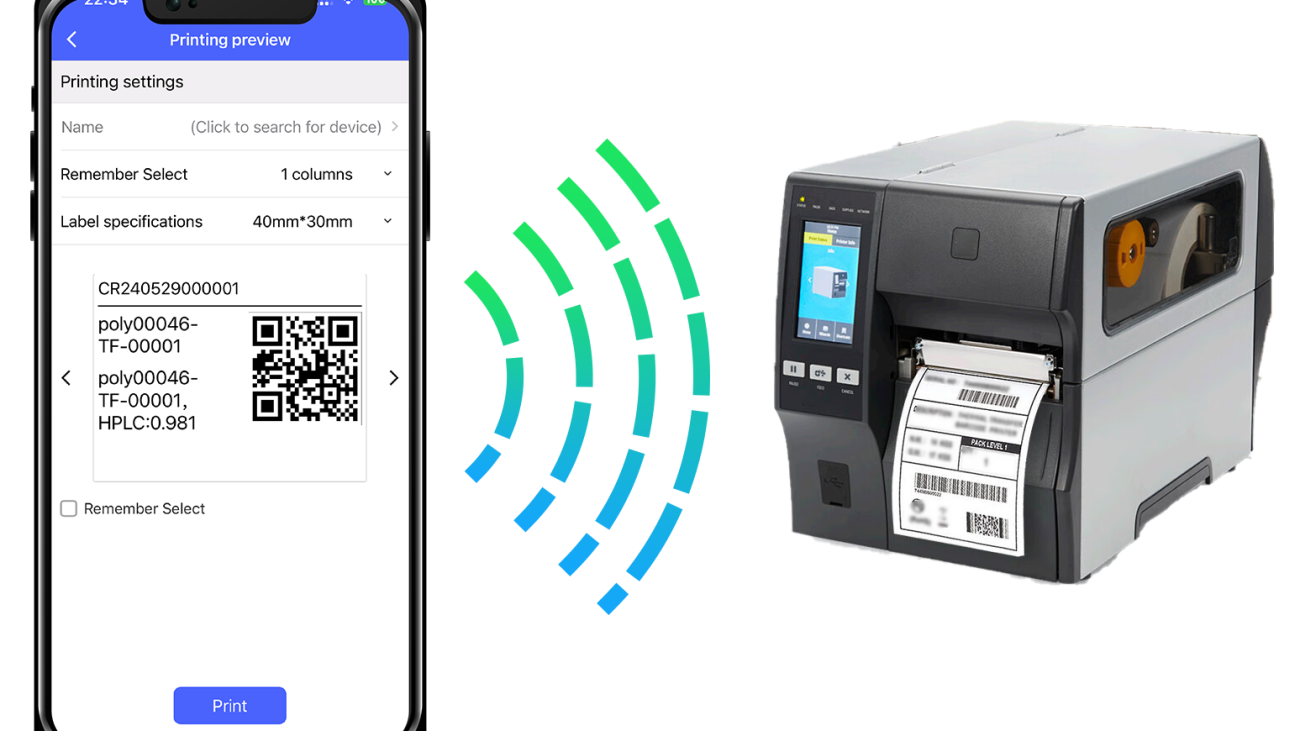
Instant Data Sync for Seamless Collaboration
Collaboration is essential in today’s research landscape, and the iLabPower ELN app ensures that our teams can work together more effectively. With instant data synchronization, we can effortlessly share and access experimental data across multiple devices in real-time. Whether we are in the laboratory, at our desks, or working remotely, the seamless connectivity provided by the ELN app allows us to remain updated on each other’s progress. This capability enhances our collaborative efforts, enabling timely feedback and the efficient execution of projects.
Comprehensive Experiment Management for Organized Research
Managing experimental records can often be a daunting task, but the iLabPower ELN app simplifies this process through comprehensive experiment management features. We can efficiently track and manage all our experimental records, ensuring that critical data is organized and easily accessible whenever needed. By maintaining a centralized repository of our research data, we minimize the risk of lost information and enhance our ability to analyze results over time. This organization is vital for maintaining the integrity of our work and aligns with our commitment to rigorous scientific practices.
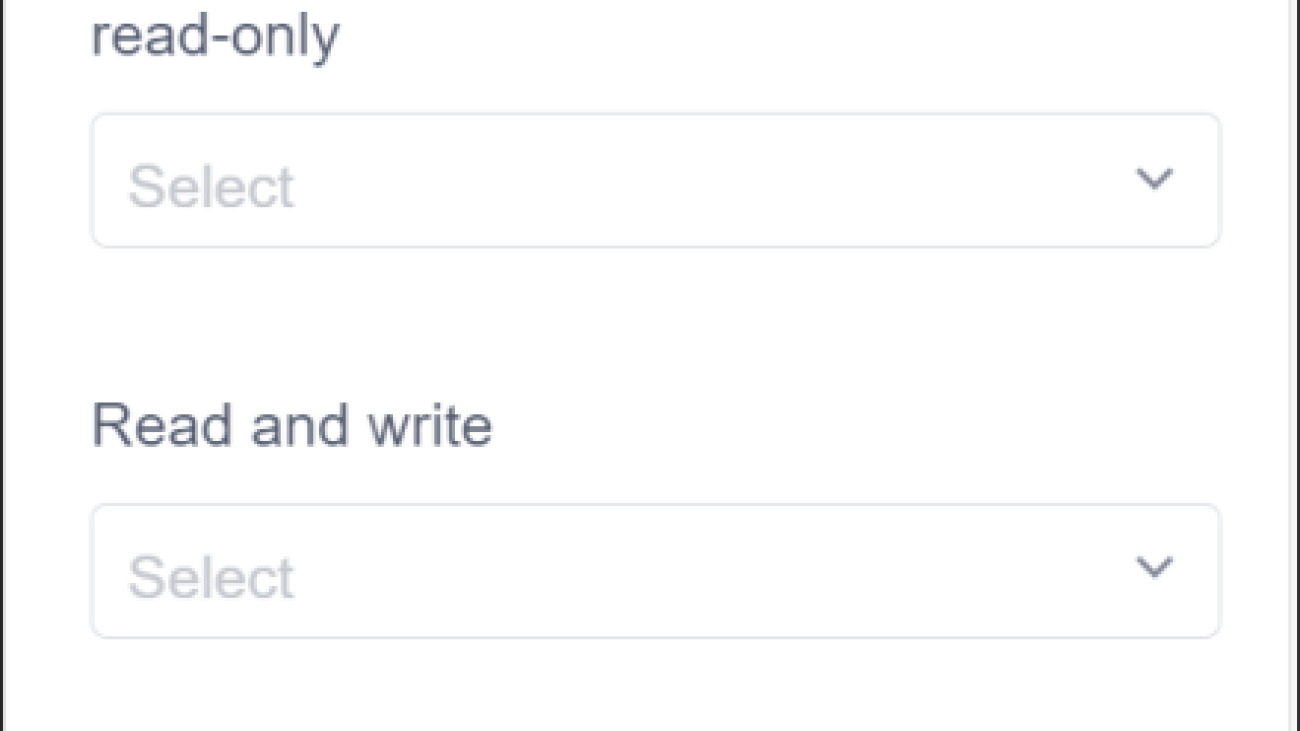
Secure Data Storage for Peace of Mind
Data security is a top priority for us, especially when it comes to sensitive experimental information. The iLabPower ELN app provides robust security protocols and secure cloud storage to protect our data against unauthorized access. With advanced encryption measures in place, we can confidently store our research findings, knowing that our intellectual property remains safeguarded. This level of security is essential in fostering a stable and trustworthy research environment.
Conclusion: Elevate Your Research with the iLabPower ELN App
The iLabPower ELN app stands as a powerful tool designed to enhance productivity and efficacy in laboratory settings. With features such as voice input support, instant data sync, comprehensive experiment management, and secure data storage, we can navigate our research endeavors with greater precision and ease.
For laboratories seeking to optimize their documentation processes and embrace modern technology in their workflows, we highly recommend considering the iLabPower ELN app from NeoTrident. Together, we can advance our research initiatives and contribute to meaningful scientific discoveries.