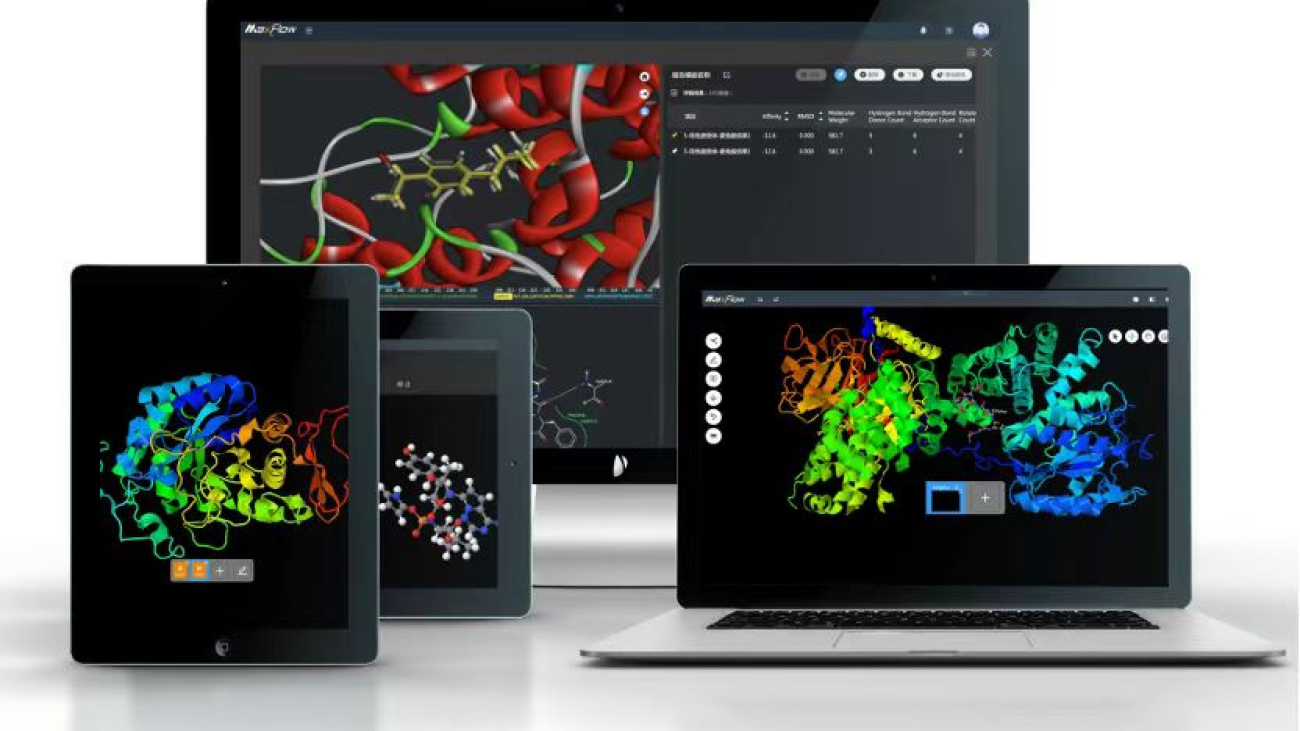
In drug discovery, the binding free energy (ΔG) between a target protein and a small molecule is a critical parameter for evaluating drug activity. Traditional computational methods, limited by insufficient sampling and model oversimplification, often struggle to balance accuracy and efficiency. The NEO-ATM module from Neotrident, leveraging advanced molecular dynamics (MD) and enhanced sampling techniques, overcomes these traditional bottlenecks to deliver high-precision, reliable relative binding free energy predictions, providing a scientific foundation for drug optimization.
Introduction of NEO-ATM
1. Relative Binding Free Energy (RBFE): Mitigating Errors in Absolute Calculations
Traditional absolute binding free energy calculations require simulating receptor conformational changes from scratch, which are prone to errors due to insufficient sampling. NEO-ATM employs relative binding free energy calculations, keeping ligands fixed within the binding pocket to avoid errors caused by receptor conformational drift, significantly improving result stability.
2. Enhanced Sampling with H-REMD: Breaking Time-Scale Barriers
Using Hamiltonian Replica Exchange Molecular Dynamics (H-REMD), NEO-ATM efficiently explores dynamic conformations of ligand binding pockets, capturing transient interactions invisible in short simulations. This reduces simulation time by over 30% while delivering results closer to experimental values.
3. Multi-Replica Parallel Computing: Reducing Statistical Uncertainty
NEO-ATM defaults to running three independent replica simulations, ensuring reliability through consistency validation. In high-variability systems (e.g., 5ns simulations), standard deviation is reduced by 40% compared to single simulations.
Case Study: Binding Free Energy Prediction for PKMYT1 Inhibitors
Background
Protein kinase PKMYT1 is a key cancer therapeutic target. Its binding mechanism with inhibitor RP-6306 directly impacts drug efficacy. Using co-crystal structures of PKMYT1 with two inhibitors (QGY, QG1; PDB ID: 8D6D/8D6E), we validated the accuracy of NEO-ATM’s ΔG predictions.
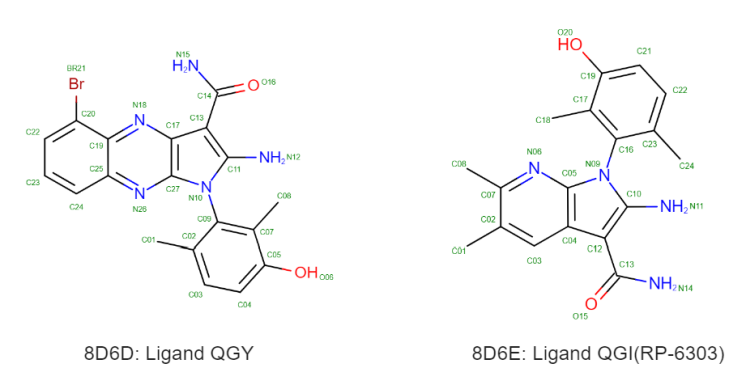
Methods
- Receptor Preparation
Protonation states optimized with PROPKA3.1; hydrogen bond networks completed via PDB2PQR.
- Ligand Preparation
Conformational optimization performed on the MaXFlow platform.
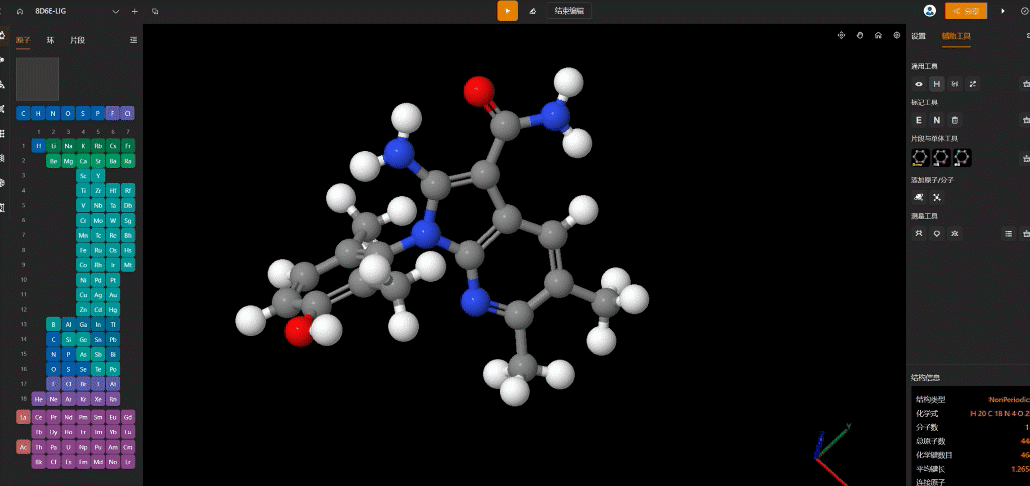
- Complex Construction
Ligands aligned to co-crystal binding sites using rigid alignment to ensure binding mode consistency.
- NEO-ATM Workflow Setup
Three replica simulations to minimize statistical error and ensure reproducibility.
- Simulation Parameters
- Results Analysis
Key Findings
- Strong Agreement with Experimental Data
NEO-ATM’s 3ns and 5ns simulations both showed excellent alignment with experimental data. RMSE and MAE fell within chemical accuracy (1 kcal/mol), with 5ns results further validating the module’s precision.
- Data Robustness Confirmed
High-quality crystal structures and experimental data were validated as reliable inputs for NEO-ATM predictions.
- Adaptability of NEO-ATM
The module excelled in capturing key target-ligand interactions for protein Y, laying a foundation for simulations without experimental baselines.
- Client Value: Accelerating Drug Discovery, Reducing Risk
Precision Optimization: Rank candidates via ΔΔG to prioritize high-affinity compounds, reducing experimental trial costs.
Early Decision-Making: Predict binding modes without experimental data, shortening lead compound discovery cycles.
Cross-Target Applicability: NEO-ATM supports over 100 protein targets, enabling rapid migration to new projects.
Empowering Innovation with Science
Creaton Technology’s NEO-ATM module provides end-to-end support for drug discovery—from target validation to optimization—through high-accuracy, efficient binding free energy calculations. Its success in the PKMYT1 case, with results closely matching experiments, demonstrates adaptability in complex systems. Moving forward, Creaton will advance AI-driven computational biology to overcome “undruggable” targets and accelerate precision medicine.
Science-Driven Intelligent Data Engine
With 20 years in pharmaceutical and materials science R&D, Neotrident empowers enterprises and research institutions to achieve digital transformation through three proprietary platforms powered by cloud computing, mobile connectivity, and scientific AI:
iLabPower R&D Platform: Manages end-to-end R&D data to ensure authenticity, integrity, and traceability. Reduces costs, boosts efficiency, and safeguards intellectual property.
SDH Scientific Data Hierarchy: Accelerates cross-source data integration and analysis to shorten time-to-market and enhance product quality.
MaXFlow Molecular Simulation & AI Platform: Democratizes molecular modeling and AI-driven innovation, replacing traditional trial-and-error approaches with data- and model-driven discovery.